





About the Clinical Study in mCSPCi
ERLEADA® + ADT was compared with placeboi+ ADT in a clinical study of 1,052 men with mCSPC
mCSPC is prostate cancer that HAS SPREAD to other parts of the body and STILL responds to medical or surgical treatment that lowers testosterone
In this study, men either received ERLEADA® 240 mg once daily or placebo. All men in the study received ADT


ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
mCSPC: Metastatic castration-sensitive prostate cancer (mCSPC) is prostate cancer that HAS SPREAD to other parts of the body and STILL responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
ERLEADA® was shown to help men live longer

In a clinical study, ERLEADA® + ADT reduced the risk of death by 35% vs placebo + ADT*
Median (middle) follow-up time was 44.0 months. In an earlier analysis from the study, the reduction in the risk of death was 33%
Here’s another way to look at the results:
At 4 years, approximately 65% of men taking ERLEADA® + ADT were alive vs 52% of men taking placebo + ADT*
Here’s another way to look at the results:
At 4 years, approximately 65% of men taking ERLEADA® + ADT were alive vs 52% of men taking placebo + ADT*
*Median (middle) data has not been reached for ERLEADA®.
ERLEADA® reduced the risk of prostate cancer getting worse

In a clinical study, ERLEADA® + ADT reduced the risk of prostate cancer getting worse by 52% vs placebo + ADT†
Here’s another way to look at the results:
At 2 years, approximately 68% of men taking ERLEADA® + ADT lived without their disease getting worse vs 48% of men taking placebo + ADT†
Here’s another way to look at the results:
At 2 years, approximately 68% of men taking ERLEADA® + ADT lived without their disease getting worse vs 48% of men taking placebo + ADT†
†Median (middle) data has not been reached for ERLEADA®.
ERLEADA® helped men achieve a zero PSA level‡

ERLEADA® + ADT helped more than twice as many men achieve a zero PSA (prostate-specific antigen) level‡ vs placebo + ADT (68% vs 32%)§
‡Zero PSA level = <0.2 ng/mL.
§The relationship between ERLEADA® and PSA is not fully known.
ERLEADA® may delay the need for chemotherapyi

ERLEADA® + ADT reduced the risk of beginning chemotherapy by 61% vs placebo + ADT║
║Median (middle) data has not been reached.
ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
Chemotherapy: Type of drug(s) that kill cancer cells.
Median: The middle number in a set of numbers. For 50% of people, this value was larger, and for 50% of people, it was smaller.
mCSPC: Metastatic castration-sensitive prostate cancer (mCSPC) is prostate cancer that HAS SPREAD to other parts of the body and STILL responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
Progression: Disease spreading further as measured by imaging studies or dying.
Prostate-specific antigen (PSA): A protein made by the prostate and found in the blood. Those with prostate cancer may have PSA blood levels that are higher than normal.
Time to chemotherapy: Length of time from when patients began study to starting chemotherapy.
About the Clinical Study in nmCRPCi
ERLEADA® + ADT was compared with placeboi + ADT in a clinical study of 1,207 men with nmCRPC
nmCRPC is prostate cancer that HAS NOT SPREAD to other parts of the body and NO LONGER responds to a medical or surgical treatment that lowers testosterone
In this study, men either received ERLEADA® 240 mg once daily or placebo. All men in the study received ADT


ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
nmCRPC: Non-metastatic castration-resistant prostate cancer (nmCRPC) is prostate cancer that HAS NOT SPREAD to other parts of the body and NO LONGER responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
ERLEADA® helped men with nmCRPC live longer without the spread of prostate cancer

In a clinical study, ERLEADA® + ADT delayed the spread of cancer to other parts of the body or death by a mediani of 24.3 months compared with placebo + ADT.
The clinical study for men with nmCRPC also evaluated:
Overall Survival: Living Longer

Progression-Free Survivali:
Delaying the Spread of Cancer

ERLEADA® and PSA response*

Nearly 2/3 of men taking ERLEADA® + ADT lowered their PSA (prostate-specific antigen) by 90% at 12 months vs none taking placebo + ADT (61% vs 0%).
*PSA response to treatment with ERLEADA® is still being studied. The relationship between ERLEADA® and PSA is not fully known.
ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
Median: The middle number in a set of numbers. For 50% of people, this value was larger, and for 50% of people, it was smaller.
nmCRPC: Non-metastatic castration-resistant prostate cancer (nmCRPC) is prostate cancer that HAS NOT SPREAD to other parts of the body and NO LONGER responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
Progression-free survival: Length of time patients lived without their prostate cancer spreading to local or distant parts of the body or death.
Prostate-specific antigen (PSA): A protein made by the prostate and found in the blood. Those with prostate cancer may have PSA blood levels that are higher than normal.
Side effects
ERLEADA® may cause serious side effects including:
Heart Disease, Stroke, or Mini-Stroke. Bleeding in the brain or blockage of the arteries in the heart or in part of the brain have happened in some people during treatment with ERLEADA® and can lead to death. Your healthcare provider will monitor you for signs and symptoms of heart or brain problems during your treatment with ERLEADA®. Call your healthcare provider or get medical help right away if you get:
Fractures and Falls. ERLEADA® treatment can cause bones and muscles to weaken and may increase your risk for falls and fractures. Falls and fractures have happened in men during treatment with ERLEADA®. Your healthcare provider will monitor your risk for falls and fractures during treatment with ERLEADA®.
Seizure. Treatment with ERLEADA® may increase your risk of having a seizure. You should avoid activities where a sudden loss of consciousness could cause serious harm to yourself or others. Tell your healthcare provider right away if you have a loss of consciousness or seizure. Your healthcare provider will stop ERLEADA® if you have a seizure during treatment.
Severe skin reactions. Treatment with ERLEADA® may cause severe skin reactions that can lead to death or be life-threatening. Stop taking ERLEADA® and tell your healthcare provider or get medical help right away if you develop any of these signs or symptoms of a severe skin reaction:

Lung problems. Treatment with ERLEADA® may cause inflammation of the lungs that can lead to death or be life-threatening. Stop taking ERLEADA® and tell your healthcare provider or get medical help right away if you develop any new or worsening symptoms of lung problems, including:
The most common side effects of ERLEADA® include:
feeling very tired
joint pain

rash
decreased appetite
fall
weight loss
weight loss
high blood pressure
hot flash
diarrhea
fracture
Your healthcare provider may reduce your dose, temporarily stop, or permanently stop treatment with ERLEADA® if you have certain side effects.
ERLEADA® may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility. Do not donate sperm during treatment with ERLEADA® and for 3 months after the last dose of ERLEADA®.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ERLEADA®.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
View all
ANDROGEN
Before treatment
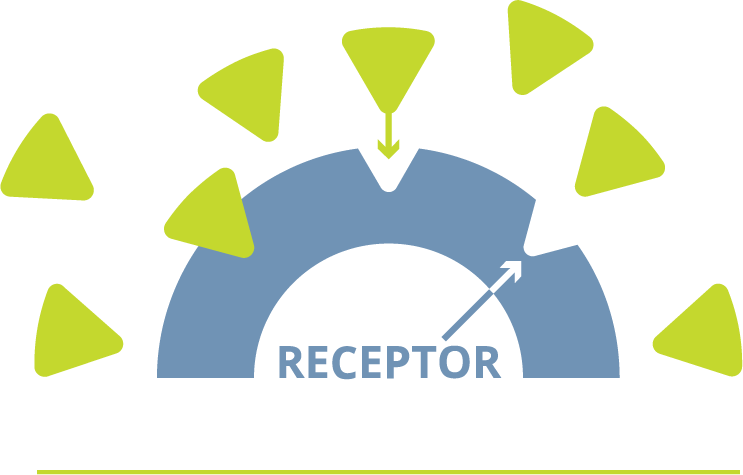
How androgens help fuel prostate cancer
Androgens are male hormones, primarily testosterone, that are needed for the prostate to function normally
However, when androgens attach to androgen receptors, they can help fuel prostate cancer cell growth
ANDROGEN REDUCTION

On ADT
The goal of ADT is to lower androgen levels
Medical or surgical treatments that lower testosterone are also referred to as androgen deprivation therapy (ADT)
ADT includes treatment to suppress or block the production or action of male hormones called androgens
ERLEADA®
ERLEADA® + ADT
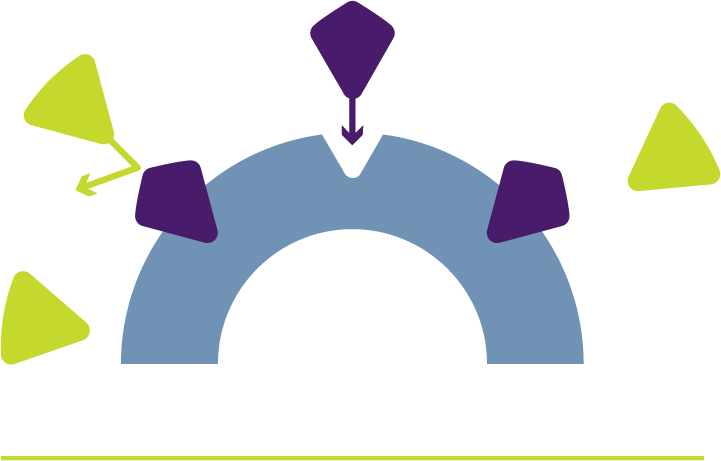
ERLEADA® + ADT fight prostate cancer together
ERLEADA® blocks androgens from attaching to receptors to help prevent cancer cells from growing
Learn more about ERLEADA® and what to expect from treatment, as well as information about available savings and support.
Always remember that your healthcare team should be your main source of treatment direction and information. An open and honest conversation can lead to a better treatment experience.


Start the conversation with your doctor about ERLEADA®. Download or print our discussion guide to bring these questions and other talking points to your next appointment.