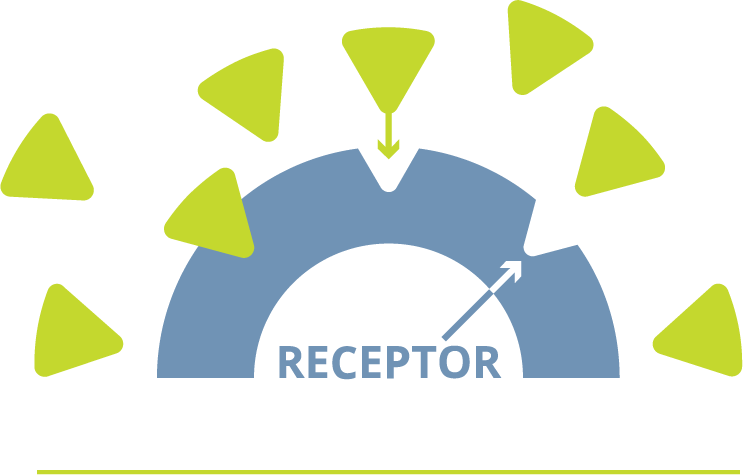
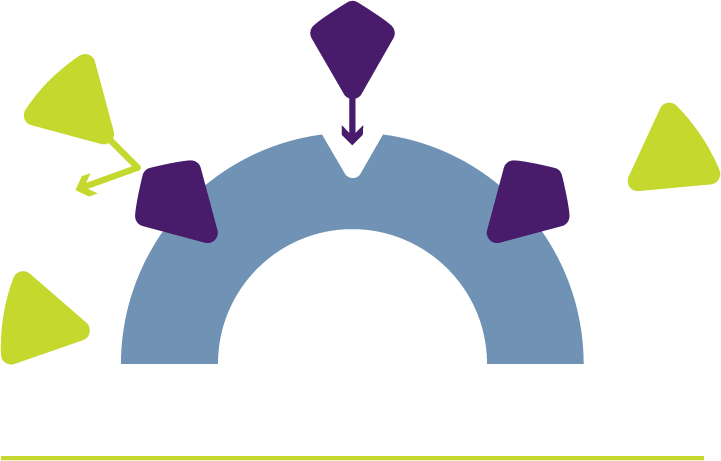
ABOUT ERLEADA® (apalutamide)
Study results in mCSPCi
ERLEADA® + ADT was compared with placeboi + ADT in a clinical study of 1052 men with mCSPC
mCSPC is prostate cancer that HAS SPREAD to other parts of the body and STILL responds to medical or surgical treatment that lowers testosterone
In this study, men either received ERLEADA® 240 mg once daily or placebo. All men in the study received ADT
ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
mCSPC: Metastatic castration-sensitive prostate cancer (mCSPC) is prostate cancer that HAS SPREAD to other parts of the body and STILL responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
Being there longer is possible™
You may live longer with ERLEADA®
In a clinical study, ERLEADA® + ADT reduced the risk of death by 35% vs placebo + ADT*
Median (middle) follow-up time was 44.0 months.
In an earlier analysis from the study, the reduction in the risk of death was 33%.
Here’s another way to look at the results:
At 4 years, approximately 65% of men taking ERLEADA® + ADT were alive vs 52% of men taking placebo + ADT*
*Median (middle) data has not been reached for ERLEADA®.
In a clinical study, ERLEADA® + ADT reduced the risk of prostate cancer getting worse by 52% vs placebo + ADT†
Here’s another way to look at the results:
At 2 years, approximately 68% of men taking ERLEADA® + ADT lived without their disease getting worse vs 48% of men taking placebo + ADT†
†Median (middle) data has not been reached for ERLEADA®.
Additional study results in mCSPC
The clinical study for men with mCSPC also evaluated time to chemotherapyi
‡Median (middle) data has not been reached.
ERLEADA® + ADT reduced the risk of beginning chemotherapy by 61% vs placebo + ADT‡
ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
Chemotherapy: Type of drug(s) that kill cancer cells.
Median: The middle number in a set of numbers. For 50% of people, this value was larger, and for 50% of people, it was smaller.
mCSPC: Metastatic castration-sensitive prostate cancer (mCSPC) is prostate cancer that HAS SPREAD to other parts of the body and STILL responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
Progression: Disease spreading further as measured by imaging studies or dying.
Time to chemotherapy: Length of time from when patients began study to starting chemotherapy.
Study results in nmCRPCi
ERLEADA® + ADT was compared with placeboi + ADT in a clinical study of 1207 men with nmCRPC
nmCRPC is prostate cancer that HAS NOT SPREAD to other parts of the body and NO LONGER responds to a medical or surgical treatment that lowers testosterone
In this study, men either received ERLEADA® 240 mg once daily or placebo. All men in the study received ADT
ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
nmCRPC: Non-metastatic castration-resistant prostate cancer (nmCRPC) is prostate cancer that HAS NOT SPREAD to other parts of the body and NO LONGER responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
Being there longer is possible™
ERLEADA® helped some men with nmCRPC live longer without the spread of prostate cancer
In a clinical study,
ERLEADA® + ADT delayed the spread of cancer to other parts of the body or death by a mediani of 24.3 months compared with placebo + ADT
Additional study results in nmCRPC
The clinical study for men with nmCRPC also evaluated:
Overall Survival: Living Longer
Progression-Free Survivali
Delaying the Spread of Cancer
Time to Chemotherapyi Delaying the Start of Chemotherapy
ERLEADA® + ADT reduced the risk of beginning chemotherapy by 37% vs placebo + ADT§
§Median (middle) data has not been reached.
ADT: Androgen deprivation therapy (ADT) includes medical or surgical treatment that lowers testosterone.
Chemotherapy: Type of drug(s) that kill cancer cells.
Median: The middle number in a set of numbers. For 50% of people, this value was larger, and for 50% of people, it was smaller.
nmCRPC: Non-metastatic castration-resistant prostate cancer (nmCRPC) is prostate cancer that HAS NOT SPREAD to other parts of the body and NO LONGER responds to medical or surgical treatment that lowers testosterone.
Placebo: Pronounced “pluh-see-bow”: a pill that looks like “real” medicine but contains nothing to affect health.
Progression-free survival: Length of time patients lived without their prostate cancer spreading to local or distant parts of the body or death.
Time to chemotherapy: Length of time from when patients began study to starting chemotherapy.
Side effects
ERLEADA® may cause serious side effects including:
Heart Disease, Stroke, or Mini-Stroke. Bleeding in the brain or blockage of the arteries in the heart or in part of the brain have happened in some people during treatment with ERLEADA® and can lead to death. Your healthcare provider will monitor you for signs and symptoms of heart or brain problems during your treatment with ERLEADA®. Call your healthcare provider or get medical help right away if you get:
- chest pain or discomfort at rest or with activity
- shortness of breath
- numbness or weakness of the face, arm, or leg, especially on one side of the body
- trouble talking or understanding
- trouble seeing in one or both eyes
- dizziness, loss of balance or coordination, or trouble walking
Fractures and Falls. ERLEADA® treatment can cause bones and muscles to weaken and may increase your risk for falls and fractures. Falls and fractures have happened in men during treatment with ERLEADA®. Your healthcare provider will monitor your risks for falls and fractures during treatment with ERLEADA®.
Seizure. Treatment with ERLEADA® may increase your risk of having a seizure. You should avoid activities where a sudden loss of consciousness could cause serious harm to yourself or others. Tell your healthcare provider right away if you have a loss of consciousness or seizure. Your healthcare provider will stop ERLEADA® if you have a seizure during treatment.

Severe skin reactions. Treatment with ERLEADA® may cause severe skin reactions that can be life‑threatening or may lead to death. Stop taking ERLEADA® and get medical help right away if you develop any of these signs or symptoms of a severe skin reaction:
- severe rash or rash that continues to get worse
- fever or flu-like symptoms
- swollen lymph nodes
- blisters or sores in the mouth, throat, nose, eyes, or genital area
- blistering or peeling of the skin
The most common side effects of ERLEADA® include:
Feeling very tired
Joint pain
Rash.
Tell your healthcare provider if you get a rash.
Decreased appetite
Fall
Weight loss
High blood pressure
Hot flash
Diarrhea
Fracture
Your healthcare provider may reduce your dose, temporarily stop, or permanently stop treatment with ERLEADA® if you have certain side effects.
ERLEADA® may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility. Do not donate sperm during treatment with ERLEADA® and for 3 months after the last dose of ERLEADA®.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ERLEADA®.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Personalized Support. From People Who Care.
Your personal Care Navigator is just a phone call away.
You can also call us at 844-NAV-1234 (844-628-1234),
Monday through Friday, 8:30 AM - 8:30 PM ET
Patient Support from Janssen Compass™